The following section is a basic introduction to RAS chemistry. It is an update of the originally leaflet – introduction to Recirculation Chemistry, written by Dr. Bent Urup back in 1997.
1) The basic principles of recirculation technology
A so called re-circulated fish farm can be many things, but in general the idea is to reuse the water a number of times.
The more treatment of the water the more times the water can be used. The optimal solution for an actual site, may depend on an economical evaluation, which will include elements as availability of water, pumping heights/costs, security of water, seaweed problems, management, optimal growth temperatures etc.
But in locations where the temperatures fluctuates a lot with low temperatures during winter and higher temperatures during summer, the benefit of obtaining a constant high constant growth rate at a constant temperature, optimal for the fish, will typically when dealing with any kind of land based farming, point towards a solution with a level of recirculation, meaning including efficient water treatment technology in enclosed indoor systems.
In the most simple systems, a certain amount of water is simply pumped back to the tanks after use with re-oxygenation as the only treatment.
More sophisticated re-circulation systems will normally include mechanical removing of particulate material (food waste and faeces from the fish), followed by removing of ammonia products, carbon-dioxide and re-oxygenation. Sometimes the systems can be supplied with bacteriological control as well, in form of UV treatment or Ozone to gain control of the dynamics of the bacteria’s.
The principal return of investments from installing complex water treatment systems, may only really be released when controlling also the temperature within the farm, maintaining an optimal temperature throughout the year. Most often this will in addition to the water treatment technology also require the fish tank and water treatment technology to be installed inside an enclosed building. Such a facility is what we by now in 2021 consider as a RAS farms.
From a simple general point of view a re-circulated fish production system will consist of a system with fish tanks from where the water is passed through a water treatment plant, where particles will be removed, new oxygen added to the water and other waste products either removed or converted to a product which is less harmful to the fish, before the water is returned to the fish-tank eventually together with some new water.
The idea is first of all that the water quality which is returned back to the fish is of a composition which will facilitate optimal growth of the fish, and that this water quality is achieved at a minimum cost.
It is implicit in this, that it is not necessarily the purpose to get the very best water quality. The important thing is to get a water quality which is good enough to keep the fish healthy, and to support an optimal growth rate of the fish kept in the system.
When dealing with commercial production, the investments and the operation costs are obviously key factors.
Applying Recirculation for aquaculture is by end of the day all about mass balances. Securing a stable environment for the fish, by installing technology which can remove the waste products from the fish in equivalent quantities as they are produced by the fish.
2) Food and energy conversion in the fish
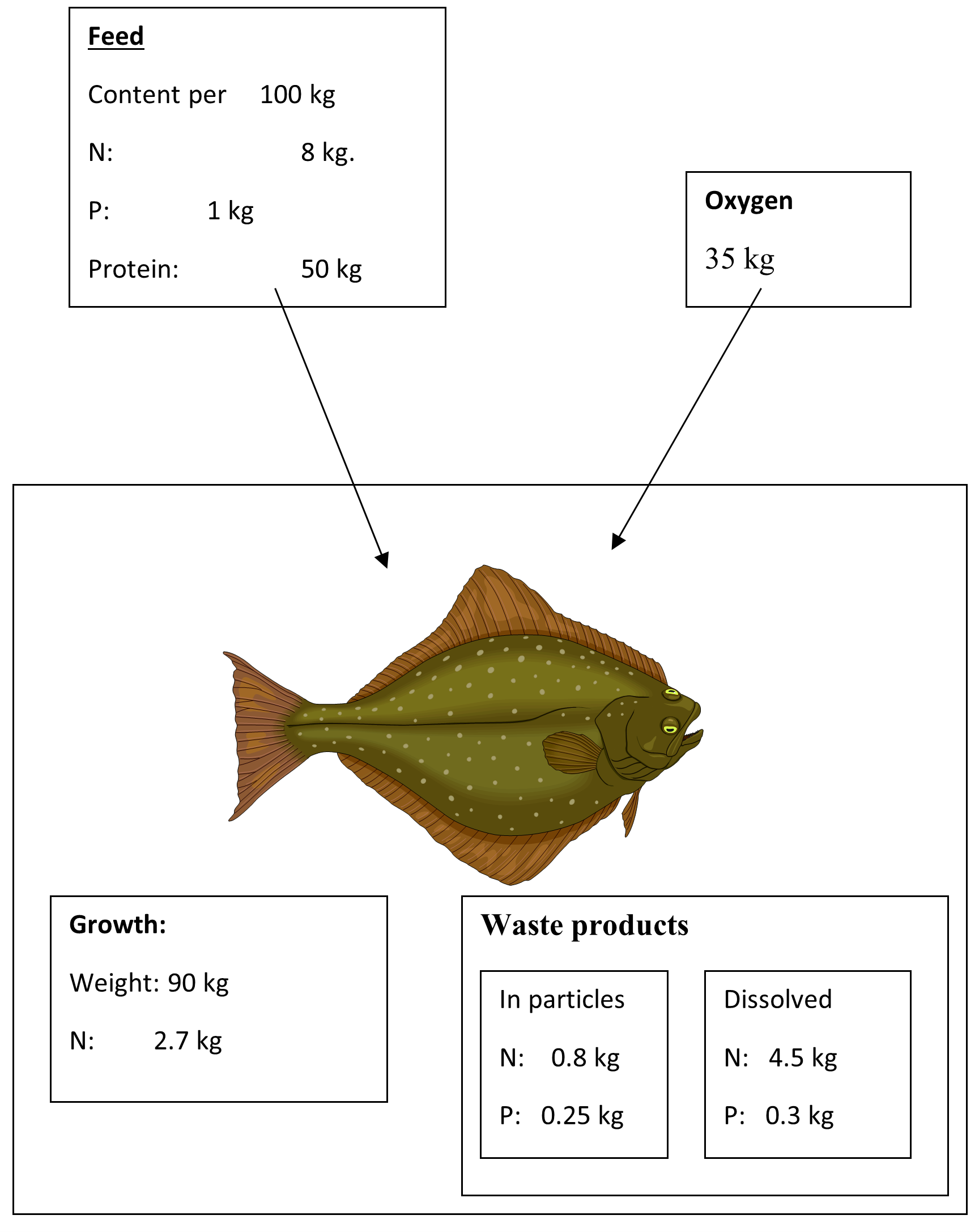
Fig. 2.1
The values are of a general character, as the actual values always will depend on a number of parameters in the actual production situation, including the fish specie which will be farmed.
A typical average feed will contain approx. 50% protein. As protein contain 16 % of nitrogen, it means that the feed will contain approx. 8% Nitrogen. The fish will contain approx. 3 % of Nitrogen compared to total wet weight.
The proportion of the feed which is not used to build new tissue in the body of the fish, will have to be released to the surrounding environment.
The same considerations is applicable in respect to phosphor. The content of phosphor in the feed will typical be in the range of 1 % while in the fish phosphor will be at a level of approx. 0.5 % of the wet weight. An exception is flatfish species, like turbot which have a higher content of phosphor – approx. 0.65%.
From figure 2.1 it can be seen that the major waste products are Phosphor, Nitrogen and carbon-dioxide.
- Nitrogen
Nitrogen will mainly be excreted by the fish as Ammonia. Approx. 80% of the ammonia excreted by the fish will be excreted above the gills, while the rest will be excreted in the faeces.
The ammonia excreted above the gills will be excreted as unionised ammonia - NH3, but due to the pH and the high pKa value of the NH3 – NH4+ buffer system, the majority will immediately be converted to NH4+ , which is not nearly as toxic to the fishes, as it do not pass well back to the fishes though the gill epithelia.
NH3 is quite toxic, and for the majority of fish-species, the level of NH3 should not be above 0.02 mg/litre under normal production conditions. Higher levels have though sometimes been seen in re-circulated system without drastically effects. There is though a high risk involved with higher levels. Further more higher levels will cause reduced growth of the fishes. Therefor the very max level of NH3(N) accepted in a RAS facility, should be 0,02 mg/l.
The problem with ammonia may call for attention not at least in seawater because of the high pH, see fig. 2.2 & fig . 2.3.
Any quick increase in pH within a production facility should be avoided.
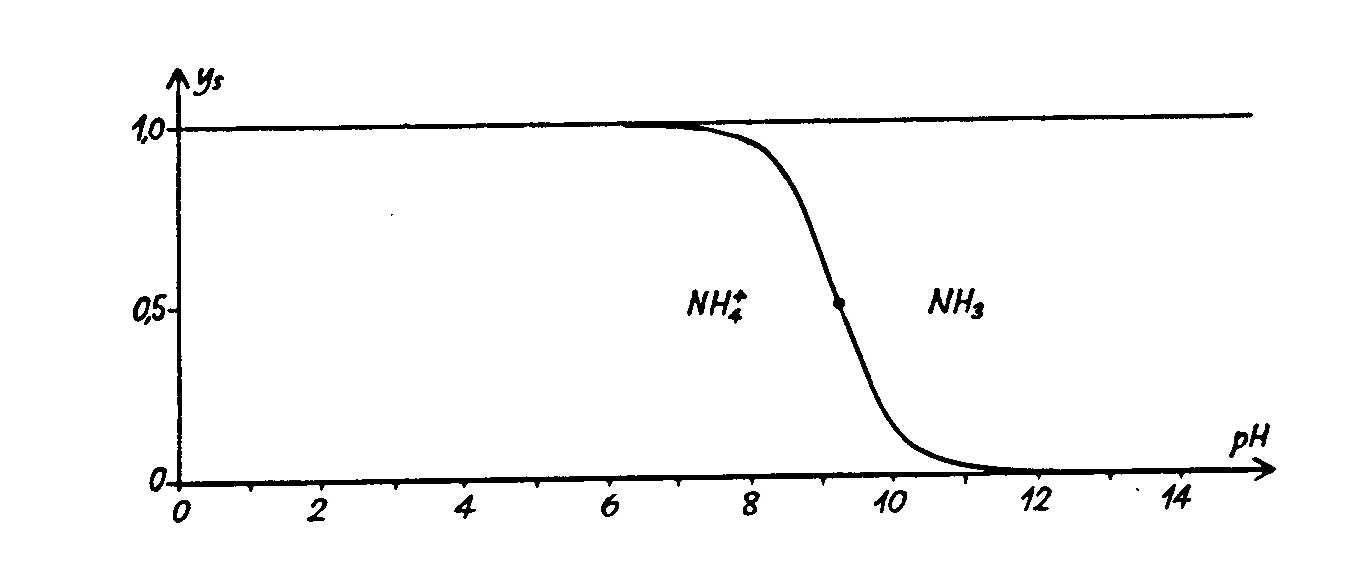
Fig. 2.2
The equilibrium is though not only depending on the pH, the salinity has some influence as well. Depending on source you will find small difference in measured amounts. See fig. 2.3,a, 2.3,b & 2.3,c below.
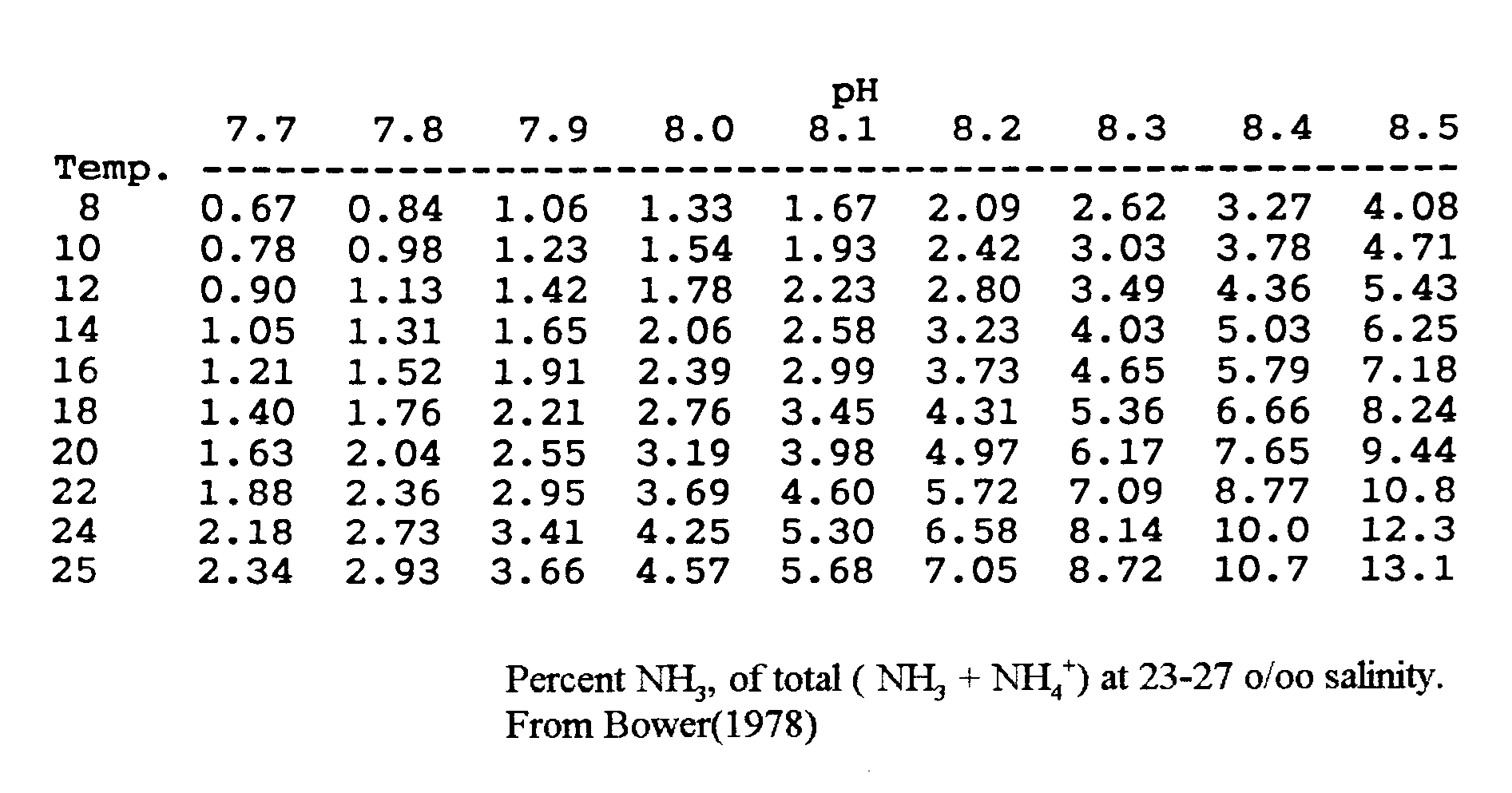
Fig. 2.3,a
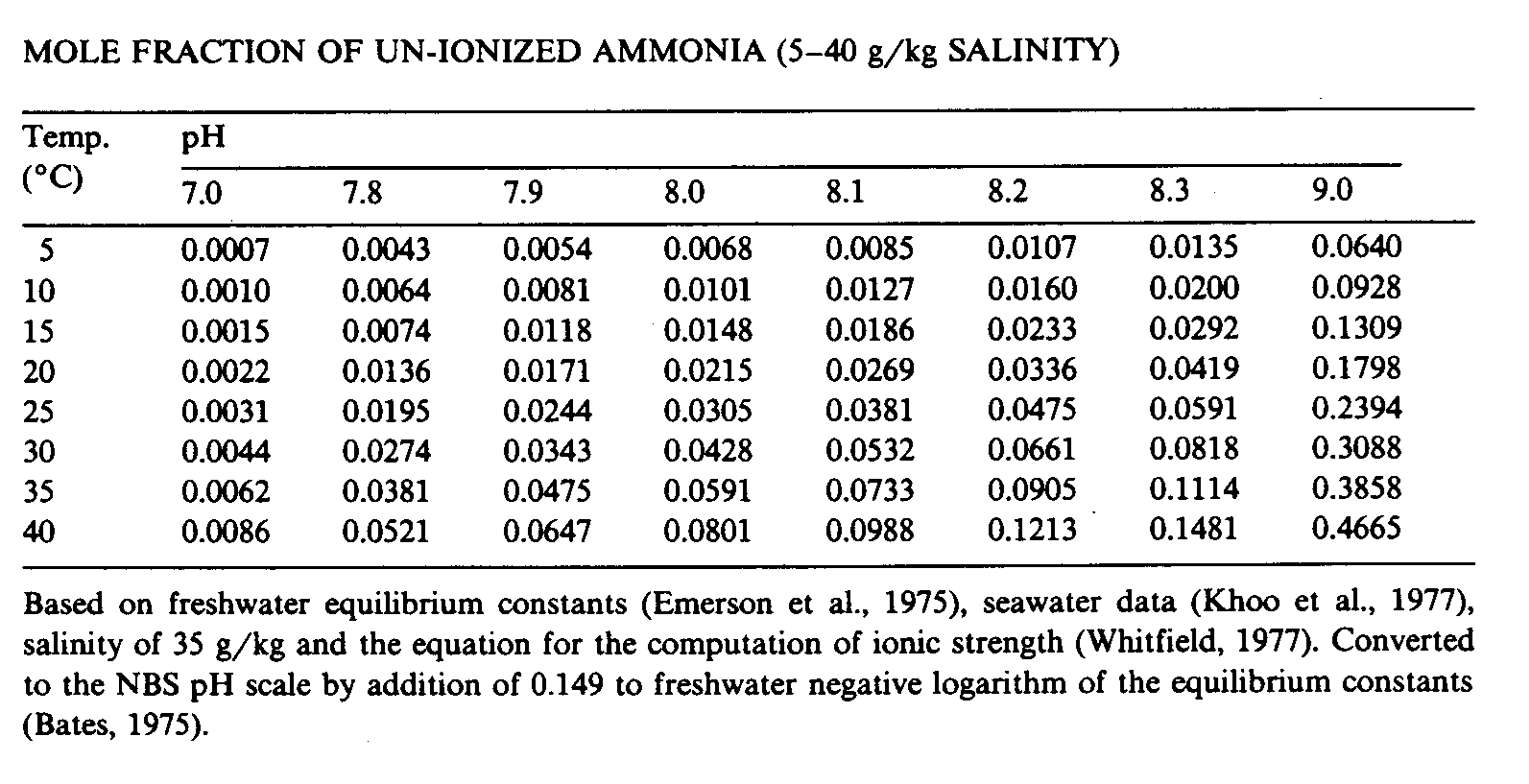
Fig. 2.3,b
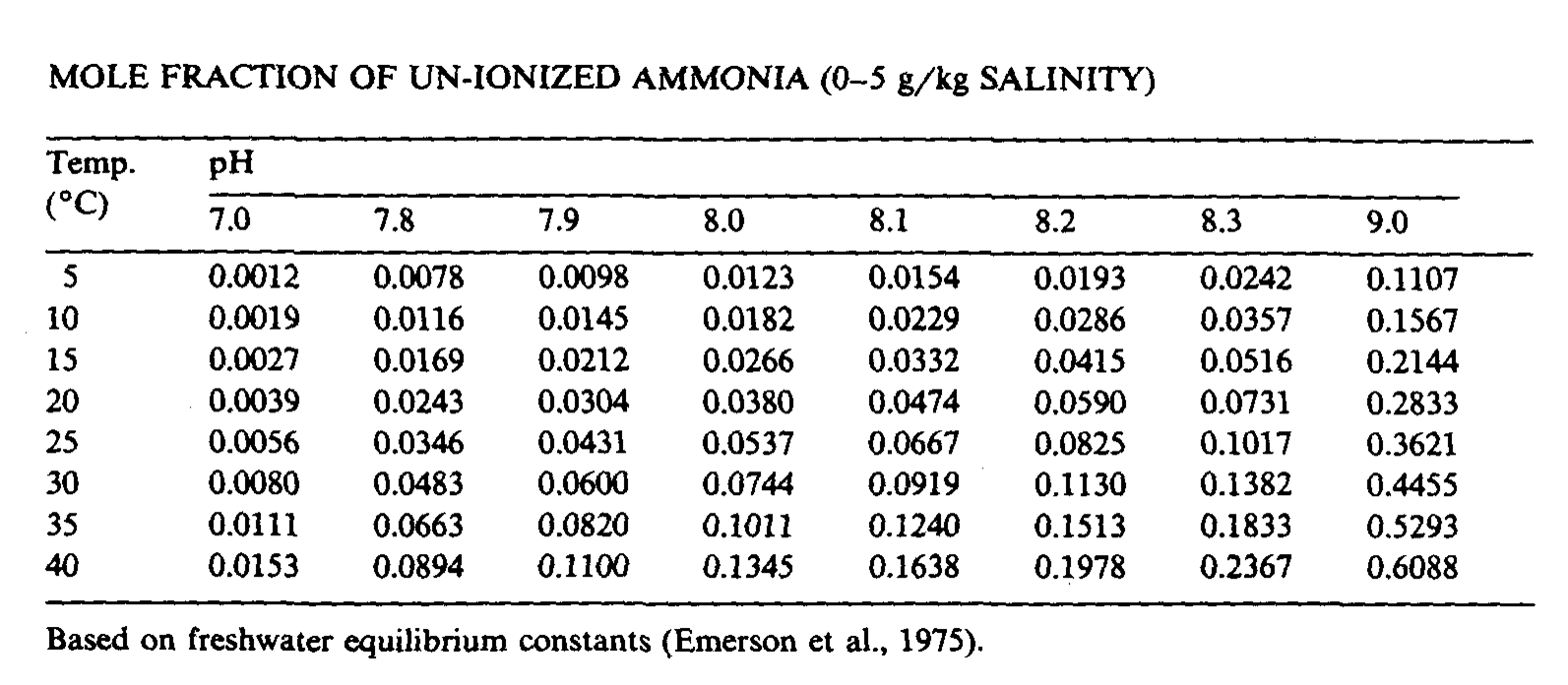
Fig. 2.3,c
- Carbon-Dioxide
The second most important waste product is carbon dioxide. It is excreted above the gills.
The fish uses Oxygen to oxidise fat, sugar and proteins, the carbon chains are broken to free energy, the carbon atoms are then releases as CO2.
At a total breakdown of fat there will be produced approx. 0.71 mole of CO2 per each used mole of O2, when proteins are the energy source 0.8 moles of CO2 will be produced, and from glucose there will be produced 1 mole of carbon dioxide for each mole of oxygen.
In seawater and RAS farms in general, we have to pay special attention to the carbon dioxide because of the pH depending chemical equilibrium with HCO3- and CO32-.
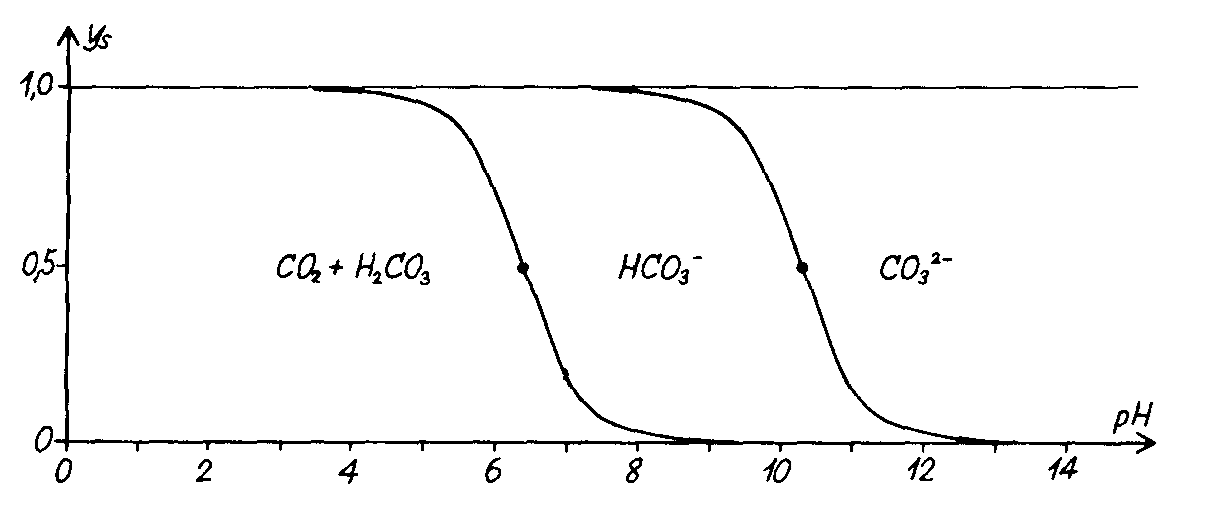
Fig. 2.4
In seawater the major part of CO2 will be transferred to HCO3-, which is not as big a problem to the fishes as CO2, it though still seems in some degree to influence the capacity of the blood in the fishes to transport the CO2 from the tissue to the gills.
The equilibrium between CO2 and HCO3- is not spontaneous, which gives some benefits but also some disadvantages in sea water..
In a pump through system including no or only a very limited reuse of the water, the carbon dioxide will be converted to HCO3- which is less harmful to the fishes. Basically the CO2 becomes diluted our of the system, without the CO2 reaching critical levels. This was in the early days of recirculation causing the misunderstanding, that CO2 was really not an issue in fish farming, and also not when dealing with recirculation.
originally
The problem is though, that as the CO2 converts to bi-carbonate, it at higher levels of reciurculation and reuse of the water, then accumulates as bicarbonate, and then becomes more difficult to get out of the water again as only the CO2 fraction can be stripped out of the water by normal means.
That means if we don’t have and effective system for removing of the carbon dioxide at the same rate as the CO2 will be excreted by the fish, then we will have a slow build up of carbon dioxide to unacceptable levels.
Optimal we in the recirculation system should keep the levels of CO2 below 10-20 mg/l.
Dealing with the CO2/bicarbonate equilibrium has clearly been one of the biggest challenges when dealing with RAS technology, especially for Seawater, as the build-up of bicarbonate will reduce the growth rate of the fish.
Discussing CO2 levels confusing can easily arise. We normally talk levels of free CO2, which may not be what we measure, depending on which technology we apy. Equipment can be bought like from Oxyguard, which can measure levels of free CO2, but otherwise total amount of CO2 included the amount bound as bicarbonate, by titration, then from the pH level calculate the fraction of CO2.
When measuring CO2 levels in the system, a measurement only mkakes any meaning i8n parallel with a pH measurement.
If measuring the free CO2 leve before and after a CO2 stripping, the free CO2 levels might well be almost similar, even if the process has removed nearly 100 % of the free CO2. But the pH will have increased, as bicarbonate have transformed back into free CO2. The actual amount of CO2 removed, then needs to be calculated from the sum of 1) The difference in from CO2 levels before and after the treatment and 2) The amount of CO2 regenerated from bicarbonate, calculated from the change in pH levels from before and after the treatment.
- Phosphorus
Some accumulation of phosphor doesn’t seem to be a problem to the fish. And in recirculation systems where foam separators are applied, it can even have some benefits in supporting the foam creation.
Apart from this, we with our present knowledge in general do not pay much attention to the phosphor level in the production water.
Regarding the discharge water from the system, phosphor is often a key component in the environmental permit. Even when the recipient is marine.
There is normally very high natural levels of phosphor in marine environments, and there for a discharge of phosphor from a RAS facility, will make no real impact on the environment, where as the opposite is the case if the recipient is a lake or a river /freshwater environments where phosphor will typically be a limiting nutrient.
It is on the other side, relatively easy to reduce the phosphor content in discharge water.
- BI5
The biological oxygen demand is a very important factor, and one of the reasons why it is important to effectively remove particulate matter in the mechanical filter. From figure 2.1 it can be seen, that we by removing particulate matter also will remove nitrogen and phosphor.
If we didn’t removed the particulate matter it alternatively had to be broken down biological in the production water, causing consumption of oxygen and production of Carbondioxide, which we then had to add/remove. It is important to notice, that the level of BI5 in the biological filter have to be relative low before it starts working for converting ammonia products. The most effective way to make the biofilter work effective is by having an effective mechanical filtration.
The introduction of effective high capacity mechanical filter systems was one of the most important break throughs for the development of recirculation systems back in the late 1980ties.
It made a dramatical increase in performance of the biofilters, and it made it possible to avoid the problems which ecto parasites previously had caused in recirculated farms and fish farms in general.
The biofilter system in. A recirculation farm without efficient mechanical filtration, might have to be 3-4 times bigger, and would further generate a lot of sludge and consume high amounts of oxygen.
The total oxygen. Consumption in a facility without efficient mechanical filtration could potentially be up to twice as high as if a very efficient mechanical filtration had been implemented.
- Oxygen
The fish uses the oxygen for oxidising the food see above under Carbon dioxide.
We can add oxygen to the fishes by adding new water with a natural oxygen content, or we can aerate the water either using air under pressure combined with diffusers at the tank bottom, or using cascades, and finally we can use pure oxygen.
The use of pure oxygen is an efficient useful source, which typically will be required in intensive fish farms. But it is a tool which requires to be managed with care , with focus on CO2 levels, as the addition of pure oxygen as an alternative to any form of aeration, will not remove any carbon dioxide, which may be as much a problem in the farm as a shortage of oxygen.
It seems that effective cascades at this stage of the art, is the most effective way to remove the carbon dioxide, and at the same time it gives an effective aeration.
Some recirculation system uses super-saturation of the production water. This should though be avoided, or applied with care, as even small levels of super-saturation (as 110% oxygen saturation), potentially will reduce the growth rate.
When it still some times can give better results with oxygen super-saturation, it is when the system is operated with to high carbon dioxide/bicarbonate levels, as these parameters interacts. It will still though give far better results if both parameters were under control.
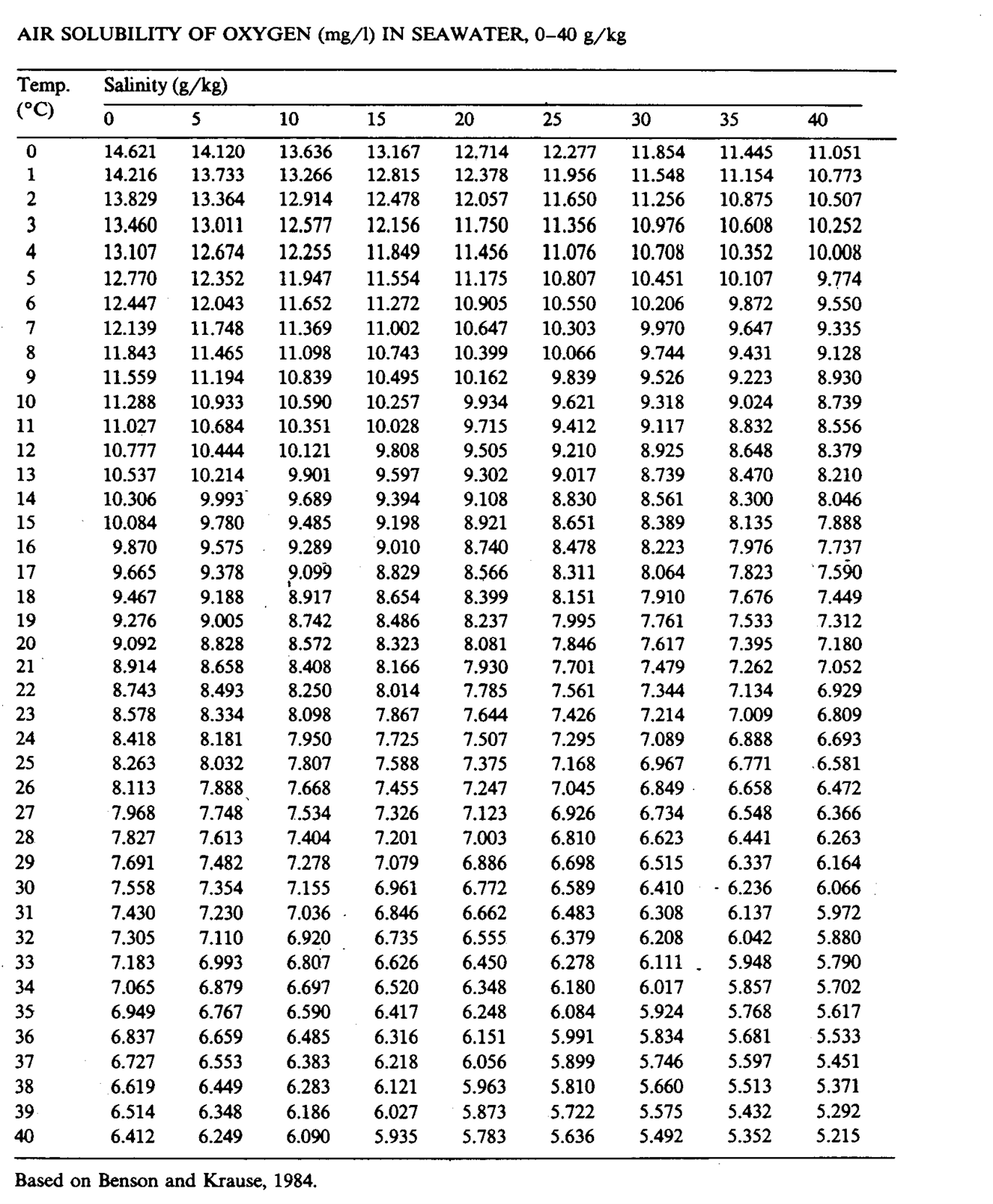
Fig. 2.5
3) The chemical parameters in the system
- Recommended general levels of compounds/parameters:
Ammonia (NH3): < 0.02 mg/l**) (<0.05mg/l)
Nitrate(NO3-): < 60 mg/l (NO3- - N)
Nitrite (NO2-): <0.1-1 mg/l (NO2- - N)*)
Carbon-dioxide(CO2): < 15 mg/l (30 mg/l)
Oxygen (O2): 70-110 % saturation.
Hydrogen Sulphide (H2S): < 0.001 mg/l
Chlorine residuals: < 0.001 mg/l
pH: 6.9 -7.2
*) Not to critical in seawater, during start of biofilter, levels of more that 8 mg/l can be found.
- Metals – recommended max levels
Aluminium (Al) < 0.06 mg/l
Cadmium (Cd) < 0.005 mg/l
Chromium (Cr) < 0.025 mg/l
Copper (Cu) < 0.005 mg/l
Iron (Fe) < 0.3 mg/l
Lead (Pb) < 0.01 mg/l
Manganese (Mn) < 0.025 mg/l
Mercury (Hg) < 0.0001mg/l
Nickel (Ni) < 0.005 mg/l
Zinc (Zn) < 0.025 mg/l
- Water Chemistry parameters
i. Ammonia (NH3 – NH4+):
Waste product from the metabolism of the fishes. Even relative small amounts of the unionised form, makes it difficult for the fish to excrete ammonia through the gills, which thereby makes even small amounts of ammonia very toxic to them.
ii. Alkalinity:
iii. Carbon dioxide(CO2)
Carbon dioxide is produced by the metabolic activity from the fishes and bacterial breakdown of organic waste products in the system. It will influence the oxygen uptake in the fishes. Levels above 20 mg/l should be avoided in the fish tanks, as higher levels will reduce growth. Optimal levels should be below 10-15 mg/l.
iv. Nitrate (NO3-)
Nitrate is formed by the nitration from ammonia to nitrite and then to nitrate. It is not directly toxic to the fish. Levels of less than 70 mg(N)/l does not seem to have negative effect on most fish-species, and some recirculation systems are operating with levels up to 250 mg(N)/l, which though is known to be lethal to some fish-species.
v. Nitrite (NO2-)
Nitrate is an intermediary product in the nitrification process of ammonia to nitrate. It is very toxic to fish, as it blocks haemoglobin in the blood for the uptake of oxygen. Levels of 0.15 mg/l have proven in cases to be total lethal in freshwater. In saltwater it is far less toxic, as it then seems to be enable then to penetrate the gills. It is recommended to keep the level below 0.1 mg/l, though variations maybe up to 1 mg/l have to be accepted. Any levels above 20 mg/l should be avoided if possible, even during up start of biofilters.
vi. Oxygen
To provide optimal and equal growth of the fishes, the oxygen level, should at any time, any place in the tank, be between 65 and 100 % saturation. It should never at any time be possible to find oxygen levels below 60 % saturation in the fish tank.
If pure oxygen is used, it is worth to notice, that any super-saturation should be avoided as even levels of 110 % saturation will cause reduced growth, and levels of 160% can be directly lethal to some fish-species.
The solubility of oxygen varies with temperature and salinity see figure 2.5.
In the biofilter there should be above 4 mg O2/l even in the outlet, to get the biofilter perform optimal. If the oxygen level drops below 2 mg there is a risk of production of toxic compounds as H2S . The nitrification will then be reduced considerable too, and the level of ammonia and nitrite will increase.
vii. pH:
pH has influence on the toxicity of other chemical compounds, beside it influences the uptake/release of carbondioxide/oxygen in the blood/tissue/gills of the fish.
From the nitrification process:
NH4+ + 1½ O2 → 2 H+ + NO2- + H2O
It can be seen that this process will reduce the pH by the production of H+ .
Also the production of carbon dioxide from metabolic activity from the fishes and bacterial breakdown of organic waste products in the system, will reduce the pH if similar amounts of CO2 as produced are not removed in the cascades. See fig. 2.4
CO2 + H2O à H2CO3 → H+ + HCO3- à2H+ + CO32-
In seawater because of the natural buffers, a sufficient water exchange will normally be enough to keep the pH within an acceptable range.
Different compounds can alternatively be used to buffer or increase the pH.
Though sodium carbonate (Na2CO3) is often used, we do not recommend to use that in seawater.
We prefer to use NaOH, when addition is required only for the purpose of pH adjustment, though of cause considerable care has to be taken then.
4) Biofilter chemistry
- Biofilter
The purpose with the biofilter is to create an environment where a biological transformation of ammonia products into nitrate can take place. The process known as nitrification. As the necessary involved bacteria’s will concentrate on surfaces, the biofilter consist of a tank with material having a high surface/ volume ratio. It is though not only the total area that counts, but also how efficient the water containing the ammonia products is distributed to the surface of the filter material.
In the first part of the filter, organic material which haven’t been filtered away in the mechanical filter will be broken down, through bacterial activity. It will inhibit the nitrification process, as the bacteria’s involved in the nitrification won’t be carrying out the nitrification as long as the organic load is to high.
The breaking down of organically compounds will consume oxygen and produce carbon dioxide, and some aeration /oxygenation is therefore needed, also in the first part of the filter.
Heterotrophic activity in the filter may build up biofilm on the biofilter media, where as the nitrification is only effective when the biofilm is very thin, preferable below 50 micron.
A visible biofilm therefor indicates a misbalance in the biofilter, that the biofilter will not perform optimal.
Generally spoken, the nitrification process can be separated into two processes:
1) NH4+ + 1½ O2 à 2 H+ + NO2- + H2O
2) NO2- + ½ O2 à NO3-
The first process is mainly carried out by a group of bacteria which we for simplicity can consider as Nitrosomonas which was the general understanding during the early days of Recirculation, even it is now recognised that it is quite a bit more complex and the process may inclyd many other bacterias. In a similar way we simplified, in line with the early understanding of biofilter performance, we consider the second process in the biofilter controlled by a group of bacteria which we may consider dominated by Nitrobacter . Both groups are kemo-autotrophic, which means that they extract energy from the above chemical reactions, and mainly use the energy to build up carbohydrates from Carbon dioxide.
Both processes requires oxygen, and to for the biofilter to perform optimally, the oxygen saturation in the entire filter should be above 3-4 mg/l.
Levels below 2 mg/l will be risky as then anaerobic processes can take place, which could result in toxic compounds – this is the more dangerous in seawater compared to freshwater, mainly because of the higher level of Sulphur (creation of H2S).
It is due to the same reason, that we do not use de-nitrification in seawater, but have to dilute the system out of problems with high nitrate levels. Or apply denitrification only for the discharge outlet from the facility, without returning the flow back into the facility, which could cause fatal consequences.
Approx. 1 g of ammonia (NH4+) can be transformed into nitrate per m2 filter surface at 20 degrees. At 10 degrees the capacity will be reduced to approx. the half, which is reduced further to the half again at 5 degree.
It has to be taken into account that the first part of the filter may not take part in the nitrification process or have reduced capacity due to organic load. The heterotrophic activity may require in the range of approx. 30 % of the total filter, even with a mechanical filtration before the fbio ilter.
The doubling time at 20 degrees is approx. 20 hours for nitrobactor, and thirty hours for nitrosomonas. At 10 degrees the doubling time is approx. 40 and 80 hours respectively.
It will normally take approx. 3-5 weeks to get the filter operating acceptable from first upstart.
During upstart of filter, first high levels of ammonia will be seen, and then there will be a high level of nitrite, before both these levels drops to acceptable levels and a steady increase in nitrate can be seen . Se figure 4.1
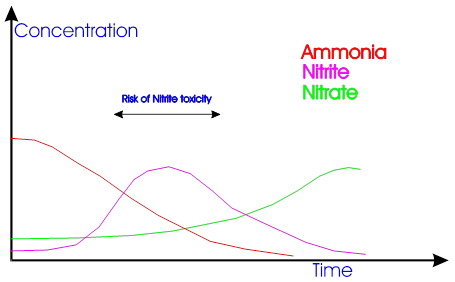
Fig. 4.1
The thinner the bacterial skin the more efficient it is. An old thick bacteria layer is inefficient and very little predictable. After first start-up of the filter, the filter will typically work most efficient approx. five weeks after the first up-start of the filter, when the filter is in routine operation, the filter will typical have its best performance approx. two weeks after each cleaning (considering a filter with a fixed filter bed).
Therefor the operation of the filter needs some working routines to maintain an efficient nitrification in the filter. The picture will vary from fish-specie to fish specie, temperatures, system set-up and other local conditions, so one will have to adjust the routines to the experience of the actual system.
